Linguistic validation & eCOA migration
in any language
Translation of Clinical Outcome Assessments and eCOA migration
Your PROs are culturally and linguistically equivalent across languages and cultures, so that your study results are comparable and meaningful.
We work with a network of vetted specialized and experienced linguists, translators, and reviewers who are native speakers of the target languages and have the required expertise in your therapeutic area.
eCOA migration: leverage the power of electronic data capture to streamline your data collection, reduce errors and missing data, and increase patient compliance and satisfaction.
We can migrate your existing paper-based PROs to the platform of your choice, or recommend a solution based on some of the following requirements:
We can also help you design custom electronic forms that are tailored to your study needs and requirements.
Linguamatics brings decades of experience managing clinical trials on the global stage, so we know how critical it is to be able to quickly enable the setting up of eCOAs.
Our eCOA library contains over 1,700 pre-configured assessments from partners who are copyrights holders, from organization and individual authors. Access to assessments that are already validated, localized and pre-approved brings immediate benefits: you can deploy studies faster, anywhere.
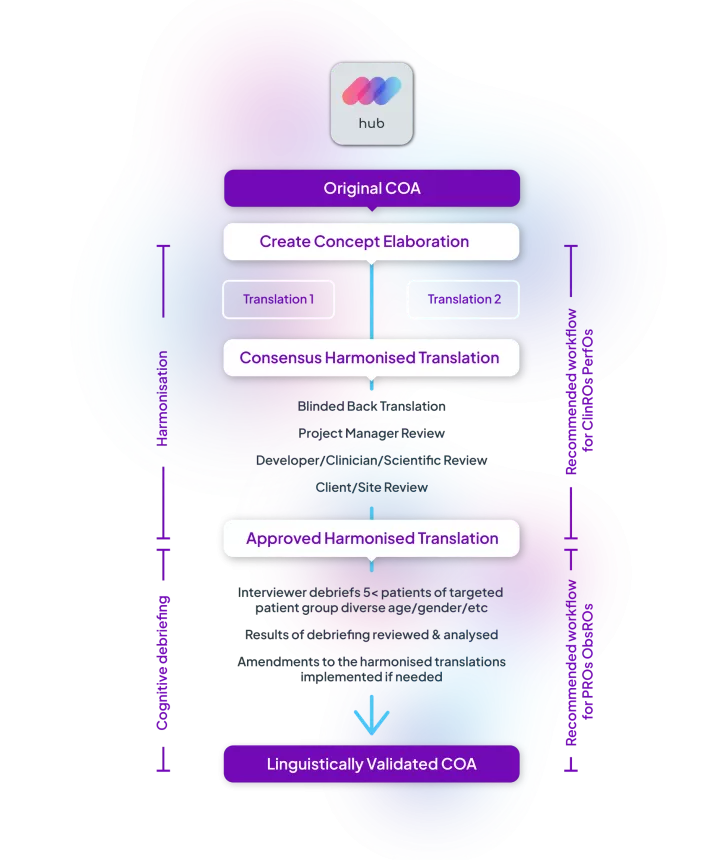
The process involves ensuring that the translated version of the instrument accurately captures the intended meaning, is culturally fitting, and is understandable to the target population.
Failure to properly linguistically validate instruments results in inaccurate data, misinterpretation of results, and ultimately compromises the scientific integrity of the trial.
Linguistic validation is crucial, and choosing a reliable provider is essential to ensure that clinical trial data is reliable, valid, and can be used to inform treatment decisions.
