Safety & pharmacovigilance
Pre-built technology and processes for quick and efficient rollout of safety monitoring across any language and regulatory bodies
Linguamatics experts have unmatched knowledge of language, culture, and regulatory requirements to enable scaling of safety monitoring documents, adverse event reports, and regulatory submissions across any languages:
Linguamatics technology suite allows safety and pharmacovigilance systems to scale across any languages easily, safely and in a timely manner
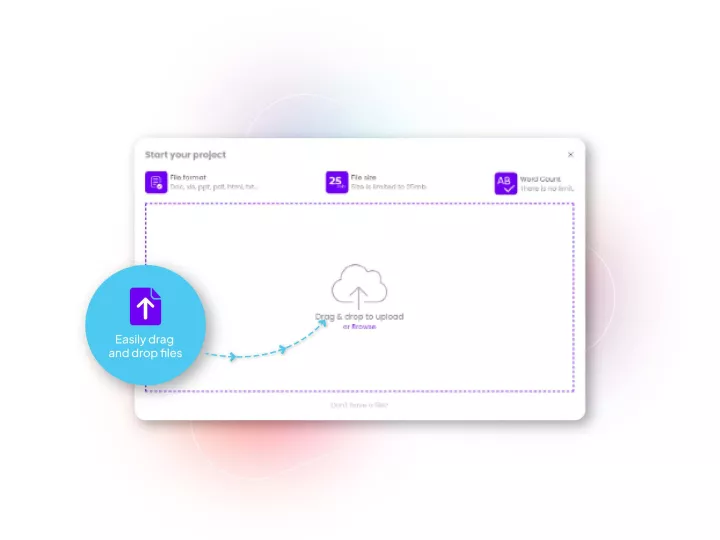
Linguamatics hub is our single entry portal allowing portal submission or system integration for sending any safety documents securely and tracking all ongoing projects and cost savings.
Our safety and pharmacovigilance workflows are pre-integrated, for easy rollout and maximum scalability.
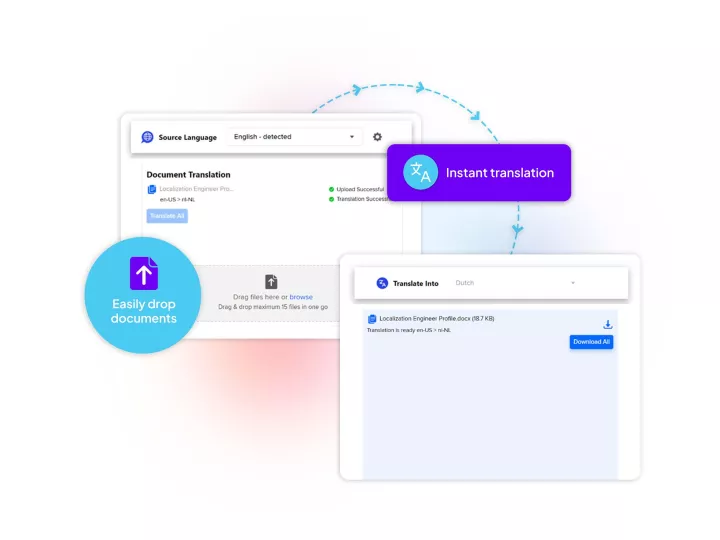
Linguamatics translate is our secured and compliant neuro machine translation solution provides the automatic translation necessary for efficient analysis and processing of pharmacovigilance data in various languages, facilitating cross-border collaboration and information-sharing among healthcare professionals, regulators, and pharmaceutical companies.
Linguamatics technology allows for faster and more accurate identification, assessment, and reporting of adverse drug reactions, ultimately enhancing patient safety on a global scale.
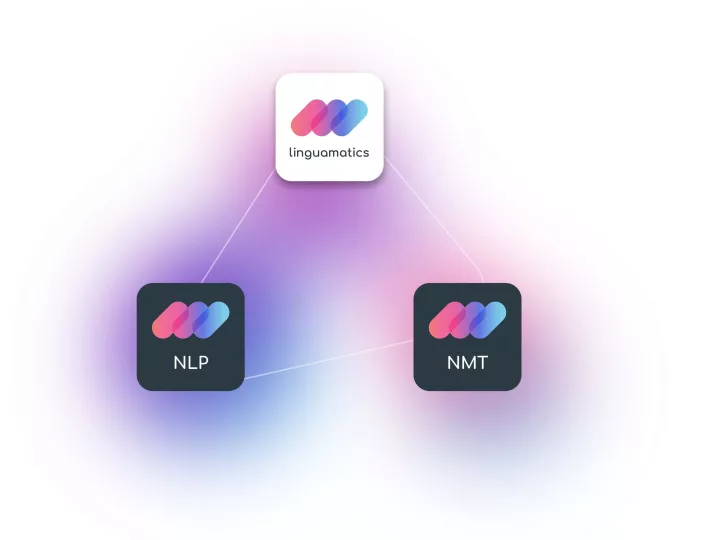
Natural language processing (NLP) algorithms and text mining techniques support the efficient analysis and processing of pharmacovigilance data.
Linguamatics NLP coupled with Linguamatics translate, enables quick identification and extraction of relevant information from large volumes of multilingual adverse event reports, enabling timely detection of potential safety concerns.

Key capabilities include:
